So I’m still just starting up, I’ve had a liquid culture of Lionsmane that has been doing well for the last 22 days (it hasn’t gone cloudy, stir it daily no complaints) it was made with a syringe I got from a Vendor. the two other strains I didn’t have any luck with, as both went cloudy and my attempts to isolate the mycelium on Agar failed.
Now 3 days ago I extracted 3 syringes of liquid culture from the lionsmane Culture, put some drops of each syringe on Agar plates, inoculated 3 jars of soaked/cooked/pressure cooked grains and injected the leftovers back into a freshly made liquid culture broth.
Looking at the newly made liquid culture broth it has gone cloudy, and looking at the petridishes I am fairly certain I am looking at bacterial contamination of the fluid.
here are the jars I’ve been using, i don’t have a self-healing injection port on there and am not using needles, instead i tàke out the filter inserted in the leurlock, screw the syringe on and inject, i do this in front of a small flow hood
the syringes don’t leave the oven bag before they are to be used in front of the flow hood,
The syringes themselves I first boil, suck up and expel boiling water 5~7 times. Then put them in an oven bag and include them in the pressure-cook alongside oven bagged Petri dishes and fresh made LC broth.
Extraction happens via the silicone tube that sticks out from the top (with its own filter) this tube goes down into the LC, When I do this I pinch off the tube as the filled syringe is decoupled from the leurlock at the end of the silicone tube
I’m not sure where Im going wrong
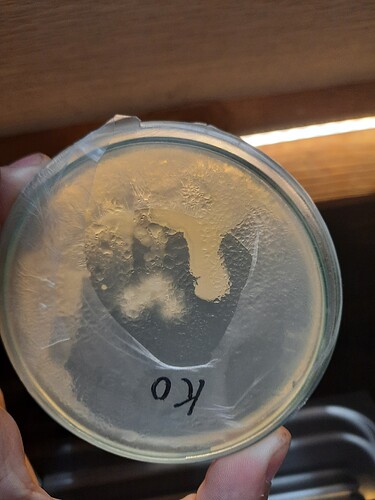
heres the agar plates, which I think indicate bacteria contamination. A small possibility is that this is just how LC broth stains the agar?
Finally, My pressure cooker only goes up to 1.2Bar, I pressure cook my liquid culture broth, syringed and petridishes for 40~45 minutes.